Sunday, April 17, 2022
Axsome Therapeutics - Near Term Catalysts Expected
Sunday, March 27, 2022
Alopecia Areata - Long Term Efficacy
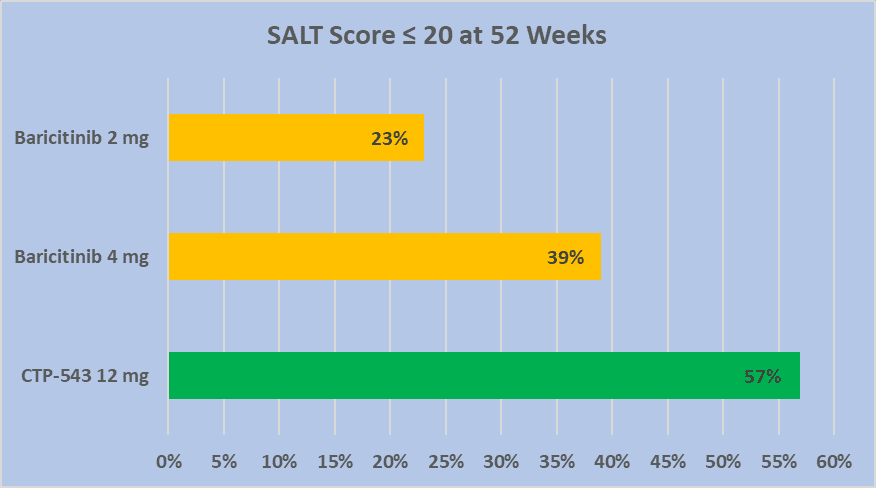
There's not an FDA approved drug, for unmet medical need Alopecia available for patients at this time. Both Lilly (Baricitinib) and Concert (CTP-543) have now released long-term (52 week) Alopecia efficacy data, using the SALT score of equal to, or less than a score of 20. Note, that a SALT score 0f zero is considered a full head of hair. So the lower the score the more hair growth has been achieved while on therapy. Below is a chart of the percentage of patients achieving the SALT score of equal to or below 20, in each Lilly and Concert's long-term clinical trials.
From the chart data above, Concerts CTP-543 has 57% of patients achieving SALT 20 or lower in their open label 52 week study. The Lilly press release of their results, can be found here Lilly. Concert’s information is located at their website under Scientific Presentations. There's a strong possibility that one, or both of these oral drugs will get approved, over the next two years. Thank you for reading.
Friday, December 31, 2021
Catalysts in 2022
Heading into 2022, there will be plenty of catalysts to look forward to. Axsome Therapeutics is in the midst of pending FDA decisions with the following.
- AXS-05 for Major Depression Disorder - Decision is forthcoming
- AXS-07 for Acute Migraine - Decision expected end of April
Additionally AXS-05 is also in a phase 3 clinical trial for the indication of Alzheimer's Agitation, with expected results in the first half of 2023. A phase 2/3 clinical trial involving AXS-05 for the indication of Smoking Cessation, is expected to be initiated in 2022. The potential approval for AXS-05 for MDD (major depressive disorder), could pave the way for future AXS-05 approvals for different indications.
Concert Pharmaceuticals CTP-543 for Alopecia Areata (an unmet need), is expected to complete at least one of the two, phase 3 clinical trials mid year 2022, with the second phase 3 completing close behind in the third quarter. These are pivotal studies that are expected to form the basis for their NDA (new drug application) for Alopecia Areata.
- Thrive AA1 phase 3 results mid year 2022
- Thrive AA2 phase 3 results 3rd quarter 2022
Thank you reading.
Thursday, November 11, 2021
Alopecia Areata - CTP-543, Baricitinib, Ritlecitinib
Of the three companies addressing the unmet medical need that exits for Alopecia Areata, both Concert Pharmaceuticals, and Lilly, have officially released SALT 20 scores. With both companies completing either phase 2 or phase 3 clinical trials, we can make comparison's from the information the companies have released to the public. Lilly has completed two phase 3's Brave-AA1, Brave-AA2 with drug Baricitinib, and Concert has completed a phase 2 with CTP-543. Both have initiated two dosing arms in their trials.
Thursday, September 16, 2021
AXS-12 for Narcolepsy
Today, Axsome Therapeutics announced that the first patient has been dosed with AXS-12 for Narcolepsy in the phase 3 clinical trial known as SYMPHONY. The expected completion, and readout of this clinical trail, will be in the first half of 2023.
AXS-07 for Acute Migraine
Wednesday, September 8, 2021
Ethereum Price Target Reached
Friday, August 13, 2021
Ethereum Price Target
The London upgrade on August 4th went smooth, and will make fees more predictable, reduce delays in processing, and decrease the amount of new coins issued. The EIP-1559 process is explained here at Cointelegraph. Ethereum is running higher after the event, with new resistance seen at 3900. Between the current price of 3290 and 3900, there are few resistance points, based on time or volume. I see the 3900 area, where the next resistance can come into play. Chart below. Thank you for reading.
Saturday, July 31, 2021
Ethereum Cryptocurrency
The second biggest Cryptocurrency, Ethereum , is about to get an upgrade that will be taking place on August 4th. The London upgrade will make fees more predictable, reduce delays in processing, and decrease the amount of new coins issued. The EIP-1559 process is explained here at Cointelegraph. Ethereum is running higher ahead of the event, with resistance at 2800 per chart below, will provide a good test at that level.
Tuesday, June 15, 2021
AXS-14 for Fibromyalgia
Another positive development involving Axsome Therapeutics and drug AXS-14, for Fibromyalgia. The FDA has given the company an expedited pathway to file a New Drug Application from the already completed, and positive phase 2 and phase 3 clinical trials. The company plans to initiate the NDA process in the fourth quarter of 2022, with expected FDA approval by third quarter 2023. This adds to their pipeline of AXS-05 for MDD, and AXS-07 for Acute Migraine. Both those drugs are already going through the FDA approval process, with AXS-05 expecting an approval by August 22nd of 2021.
Thursday, May 20, 2021
Ethereum
Monday, April 26, 2021
AXS-05 Priority Review for MDD